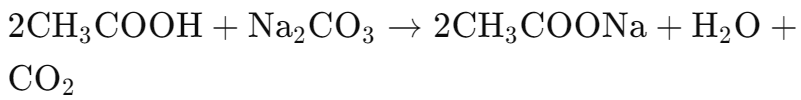Table of Contents (tap to open/close)
Carbon and Its Compounds
In this chapter, we’ll learn about carbon, an element that’s super important in both its pure form and combined form.
Activity 4.1 (Click here)
- Make a List: Write down ten things you’ve used or consumed since the morning.
- Class List: Combine your list with your classmates’ lists.
- Sort Items: Sort the items into a table, noting if they contain more than one material.
Observation: Most items in the last column of your table will be made of carbon compounds.
Questions to Consider:
- How can we test if something contains carbon?
- What happens when a carbon compound is burnt?
- Do you know a test to confirm this?
Interesting Fact:
- Food, clothes, medicines, and books are all based on carbon.
- All living things are carbon-based.
- Earth’s crust has 0.02% carbon, and the atmosphere has 0.03% carbon dioxide.
- Despite this small amount, carbon is extremely important.
Bonding in Carbon – The Covalent Bond
Recap from Previous Chapter:
- Ionic compounds have high melting and boiling points and conduct electricity when dissolved or molten.
Carbon Compounds:
- Most carbon compounds do not conduct electricity.
- They have low melting and boiling points compared to ionic compounds.
- This suggests that the attraction forces between molecules are weak and there’s no formation of ions.
Electronic Configuration:
- Atomic number of carbon: 6
- Carbon has 4 valence electrons.
Reactivity of Carbon:
- Carbon needs to gain or lose 4 electrons to achieve a stable configuration (like noble gases).
Challenges:
- Gaining 4 electrons: Forms C4- anion, which is hard because 6 protons would need to hold onto 10 electrons.
- Losing 4 electrons: Forms C4+ cation, which is hard because it requires a lot of energy to remove 4 electrons.
Solution – Covalent Bonding:
- Carbon shares its valence electrons with other atoms (carbon or other elements) to achieve a stable configuration.
- Shared electrons count towards the outer shells of both atoms involved.
Simple Molecules of Carbon and Covalent Bonds
Carbon forms simple molecules by sharing electrons, leading to stable configurations for all atoms involved.
Hydrogen Molecule (H₂)
- Atomic Number: 1
- Electrons in K Shell: 1
- Bond Formation: Two hydrogen atoms share their electrons to form H₂, achieving a stable configuration like helium (2 electrons in K shell).
- Bond Type: Single covalent bond (represented by a line between the atoms).
Chlorine Molecule (Cl₂)
- Atomic Number: 17
- Electronic Configuration: 2, 8, 7
- Valence Electrons: 7
- Bond Formation: Two chlorine atoms share their electrons to form Cl₂.
- Dot Structure: Only valence electrons are shown in the electron dot structure.
Oxygen Molecule (O₂)
- Atomic Number: 8
- Electrons in L Shell: 6
- Bond Formation: Each oxygen atom shares two electrons, forming O₂.
- Bond Type: Double bond (two shared pairs of electrons).
Water Molecule (H₂O)
- Composition: One oxygen atom and two hydrogen atoms.
- Bond Type: Single covalent bonds between oxygen and each hydrogen.
Nitrogen Molecule (N₂)
- Atomic Number: 7
- Electronic Configuration: 2, 5
- Bond Formation: Each nitrogen atom shares three electrons, forming N₂.
- Bond Type: Triple bond (three shared pairs of electrons).
Ammonia Molecule (NH₃)
- Composition: One nitrogen atom and three hydrogen atoms.
- Bond Type: Single covalent bonds.
Methane (CH₄)
- Composition: One carbon atom and four hydrogen atoms.
- Bond Formation: Carbon shares its four electrons with four hydrogen atoms.
- Valency: Carbon is tetravalent (4 valence electrons).
Covalent Bonds
- Definition: Formed by sharing an electron pair between atoms.
- Properties:
- Strong bonds within molecules.
- Weak intermolecular forces.
- Low melting and boiling points.
- Poor conductors of electricity.
Allotropes of Carbon
Diamond
- Structure: Each carbon atom bonded to four other carbon atoms in a 3D structure.
- Properties: Hardest known substance.
Graphite
- Structure: Each carbon atom bonded to three other carbon atoms in hexagonal layers.
- Properties: Smooth, slippery, and a good conductor of electricity.
Fullerenes
- Structure: Carbon atoms arranged in shapes like a football (C-60).
- Named After: Buckminster Fuller, the architect who designed geodesic domes.
Synthetic Diamonds
- Creation: Made by subjecting pure carbon to high pressure and temperature.
- Comparison: Similar to natural diamonds.
Versatile Nature of Carbon
Carbon’s Unique Properties
- Carbon forms millions of compounds, more than any other element.
- This is due to two key properties: catenation and tetravalency.
1. Catenation
- Definition: Carbon’s ability to form bonds with other carbon atoms.
- Forms: Long chains, branched chains, rings.
- Bonds: Single, double, triple.
- Saturated Compounds: Only single bonds.
- Unsaturated Compounds: Double or triple bonds.
- Strength: Carbon-carbon bonds are strong and stable.
- Comparison: Silicon also forms chains but only up to seven or eight atoms and these compounds are very reactive.
2. Tetravalency
- Definition: Carbon’s ability to form four bonds with other atoms.
- Bonding: Can bond with carbon or other elements like oxygen, hydrogen, nitrogen, sulfur, and chlorine.
- Stability: Bonds are strong due to carbon’s small size, allowing the nucleus to hold shared electrons tightly.
- Comparison: Bonds formed by larger atoms are weaker.
Organic Compounds
- Characteristics: Formed due to tetravalency and catenation.
- Examples: Many compounds have the same non-carbon atom/group attached to different carbon chains.
- Historical Belief: Originally thought to be formed only in living systems, requiring a ‘vital force’.
- Disproved: Friedrich Wöhler synthesized urea from ammonium cyanate in 1828.
- Study: Organic chemistry focuses on carbon compounds, excluding carbides, oxides of carbon, carbonates, and bicarbonates.
Saturated and Unsaturated Carbon Compounds
Saturated Compounds
- Example: Methane (CH₄) and Ethane (C₂H₆).
- Formation:
- Link carbon atoms with single bonds.
- Use hydrogen atoms to satisfy remaining valencies.
- Structure of Ethane:
- Step 1: Link two carbon atoms with a single bond: C—C.
- Step 2: Each carbon bonds with three hydrogen atoms.
- Electron Dot Structure: Shows valence electrons shared between carbon and hydrogen.
- Properties: Less reactive.
Unsaturated Compounds
- Example: Ethene (C₂H₄) and Ethyne (C₂H₂).
- Formation:
- Ethene: Double bond between two carbon atoms to satisfy valencies.
- Ethyne: Triple bond between two carbon atoms to satisfy valencies.
- Properties: More reactive due to double or triple bonds.
Chains, Branches, and Rings
Chains
- Examples: Methane, Ethane, Propane (1, 2, and 3 carbon atoms).
- Long Chains: Carbon atoms can form long chains.
Branches
- Structural Isomers:
- Example with Butane (C₄H₁₀): Two different carbon skeletons possible.
- Same molecular formula but different structures.
Rings
- Example: Cyclohexane (C₆H₁₂) forms a ring structure.
- Saturated and Unsaturated:
- Cyclohexane is saturated.
- Benzene (C₆H₆) is unsaturated with alternating double bonds.
Hydrocarbons
- Definition: Compounds containing only carbon and hydrogen.
- Types:
- Alkanes: Saturated hydrocarbons (single bonds).
- Alkenes: Unsaturated hydrocarbons (one or more double bonds).
- Alkynes: Unsaturated hydrocarbons (one or more triple bonds).
Will You Be My Friend?
Carbon Bonds with Other Elements
- Carbon can bond with elements like halogens, oxygen, nitrogen, and sulfur.
- These elements replace hydrogen in hydrocarbons, maintaining carbon’s valency.
- Heteroatoms: Elements that replace hydrogen in hydrocarbons.
- Functional Groups: Groups of atoms that replace hydrogen, giving specific properties to the compound (see Table 4.3 for examples).
Homologous Series
Definition
- A series of compounds where the same functional group substitutes for hydrogen in a carbon chain.
- Example: Alcohols like CH₃OH, C₂H₅OH, C₃H₇OH, and C₄H₉OH have similar chemical properties.
Characteristics
- Compounds in a homologous series differ by a -CH₂- unit.
- Physical Properties: Show a gradation (e.g., increasing melting and boiling points).
- Chemical Properties: Determined by the functional group and remain similar.
Activity
- Calculate differences in formulae and molecular masses for a set of alcohols.
- Identify similarities and arrange in order of increasing carbon atoms.
Nomenclature of Carbon Compounds
Naming Rules
- Identify Carbon Atoms: Name the compound based on the number of carbon atoms (e.g., three carbon atoms = propane).
- Functional Groups: Indicated by a prefix or suffix.
- If the suffix starts with a vowel, remove the final ‘e’ from the carbon chain name and add the suffix.
- Example: Propane with a ketone group becomes propanone.
- Unsaturated Compounds:
- For double bonds, change ‘ane’ to ‘ene’ (e.g., propane → propene).
- For triple bonds, change ‘ane’ to ‘yne’ (e.g., propane → propyne).
Chemical Properties of Carbon Compounds
1. Combustion
- Carbon Combustion: All forms of carbon burn in oxygen to produce carbon dioxide, heat, and light.
- Example reactions:
- C+O2→CO2+heat and light
- CH4+O2→CO2+H2O+heat and light
- CH3CH2OH+O2→CO2+H2O+heat and light
- Example reactions:
Activity 4.3: Observing Combustion
- Burn carbon compounds like naphthalene, camphor, and alcohol.
- Observe:
- Nature of flame
- Smoke production
- Deposition on a metal plate above the flame
Activity 4.4: Flames and Smoke
Bunsen Burner Experiment:
- Adjust air hole to get different flames.
- Yellow, sooty flame: Less air, incomplete combustion.
- Blue flame: More air, complete combustion.
Clean and Sooty Flames
- Saturated Hydrocarbons: Clean flame.
- Unsaturated Hydrocarbons: Yellow flame with black smoke (sooty deposit).
- Incomplete Combustion: Even saturated hydrocarbons can give a sooty flame if air supply is limited.
Pollution from Combustion
- Fuels like coal and petroleum contain nitrogen and sulfur.
- Combustion forms oxides of sulfur and nitrogen, major pollutants.
Why Do Substances Burn with or without a Flame?
- Gaseous Substances: Produce flame when burning.
- Solid Fuels (coal/wood): May glow red without a flame due to the absence of volatile substances.
Formation of Coal and Petroleum
- Coal: Formed from ancient trees, ferns, and plants compressed by earth layers.
- Petroleum: Originates from tiny sea plants and animals, transformed by bacteria under high pressure into oil and gas.
2. Oxidation
Activity 4.5: Oxidation of Ethanol
- Materials Needed: Ethanol, alkaline potassium permanganate, water bath.
- Steps:
- Warm 3 mL of ethanol in a test tube using a water bath.
- Add a 5% solution of alkaline potassium permanganate drop by drop.
- Observe if the color persists initially and what happens when excess is added.
- Explanation:
- Potassium permanganate is an oxidizing agent.
- It oxidizes ethanol to acetic acid:
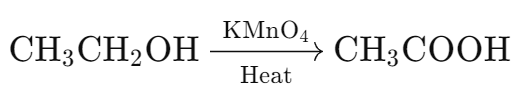
- Oxidizing agents like potassium permanganate and potassium dichromate add oxygen to substances.
3. Addition Reaction
- Unsaturated Hydrocarbons: Can add hydrogen in the presence of catalysts like palladium or nickel to form saturated hydrocarbons.
- Example: Hydrogenation of vegetable oils:
- Vegetable oils (unsaturated) + Hydrogen + Nickel Catalyst → Saturated fats
- Healthy Oils: Unsaturated fats in vegetable oils are healthier than saturated fats in animal fats.
4. Substitution Reaction
- Saturated Hydrocarbons: Generally unreactive but react quickly with chlorine in sunlight.
- Example: Methane reacts with chlorine:

- Substitution Reaction: Chlorine replaces hydrogen atoms one by one.
Some Important Carbon Compounds – Ethanol and Ethanoic Acid
1. Properties of Ethanol
- Ethanol (Alcohol): A liquid at room temperature.
- Uses: Found in alcoholic drinks, tincture iodine, cough syrups, and tonics.
- Solubility: Mixes with water in all proportions.
- Effects of Consumption:
- Small amounts cause drunkenness.
- Pure ethanol (absolute alcohol) can be lethal in small quantities.
- Long-term use leads to health issues.
Reactions of Ethanol
- Reaction with Sodium:
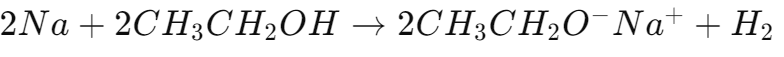
- Produces sodium ethoxide and hydrogen gas.
- Dehydration to Ethene:
- Heating ethanol with concentrated sulfuric acid at 443 K:
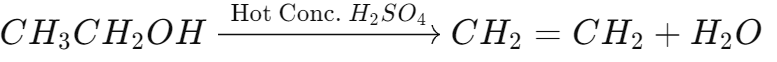
- Sulfuric acid acts as a dehydrating agent, removing water from ethanol to form ethene.
Interesting Facts
- Effects on Living Beings:
- Large quantities slow down metabolism and depress the central nervous system.
- Causes lack of coordination, confusion, drowsiness, and impaired judgement.
- Methanol, unlike ethanol, is highly toxic and can cause blindness or death.
- Denatured Alcohol:
- Industrial ethanol is made undrinkable by adding poisonous substances like methanol.
- Dyes are added to make it blue for easy identification.
- Alcohol as a Fuel:
- Sugarcane is efficient in converting sunlight into chemical energy.
- Sugarcane juice can be fermented to produce ethanol.
- Some countries add ethanol to petrol as a cleaner fuel, producing only CO2 and water when burnt.
2. Properties of Ethanoic Acid
Ethanoic Acid Overview
- Common Name: Acetic acid.
- Group: Carboxylic acids.
- Vinegar: A 5-8% solution of acetic acid in water, used as a preservative in pickles.
- Melting Point: 290 K; it can freeze in cold climates, earning the name glacial acetic acid.
- Acidic Nature: Weak acid compared to strong mineral acids like HCl.
Activities
Activity 4.7: Comparing Acids
- Materials: Dilute acetic acid, dilute hydrochloric acid, litmus paper, universal indicator.
- Steps:
- Compare the pH of both acids.
- Use litmus paper and universal indicator.
- Observations: Both acids will turn litmus paper red, but the universal indicator will show that HCl is a stronger acid than acetic acid.
Activity 4.8: Ester Formation
- Materials: 1 mL ethanol, 1 mL glacial acetic acid, a few drops of concentrated sulfuric acid, water bath.
- Steps:
- Mix the substances and warm for five minutes.
- Pour the mixture into a beaker with water and smell.
- Observations: A sweet-smelling substance (ester) is formed.
Reactions of Ethanoic Acid
- Esterification Reaction:
- Reaction: Ethanoic acid reacts with ethanol in the presence of an acid catalyst to form an ester.
- Products: Esters are sweet-smelling, used in perfumes and as flavoring agents.
- Reaction: Ethanoic acid reacts with ethanol in the presence of an acid catalyst to form an ester.
- Saponification Reaction:
- Reaction: Esters react with sodium hydroxide (NaOH) to produce alcohol and the sodium salt of carboxylic acid.
- Uses: This reaction is used to make soap.
- Reaction: Esters react with sodium hydroxide (NaOH) to produce alcohol and the sodium salt of carboxylic acid.
- Reaction with Base:
- Reaction: Ethanoic acid reacts with sodium hydroxide to form sodium acetate and water.
- Reaction: Ethanoic acid reacts with sodium hydroxide to form sodium acetate and water.
- Reaction with Carbonates and Hydrogencarbonates:
1. Activity 4.9: Observing Reactions
- Materials: Sodium carbonate, dilute ethanoic acid, lime-water.
- Steps:
- Add sodium carbonate to ethanoic acid and observe the gas produced.
- Pass the gas through lime-water to identify it.
- Observations: The reaction produces carbon dioxide (CO₂), which turns lime-water milky.
- Reactions:
- With sodium carbonate:
- With sodium hydrogencarbonate:
- With sodium carbonate:
Soaps and Detergents
Activity 4.10: Effect of Soap in Cleaning
- Materials:
- Two test tubes with 10 mL water each
- Cooking oil
- Soap solution
- Steps:
- Add a drop of oil to both test tubes (label as A and B).
- Add a few drops of soap solution to test tube B.
- Shake both test tubes vigorously.
- Observe the separation of oil and water layers.
- Observation:
- Soap helps mix oil and water, forming an emulsion.
- Without soap, oil and water separate quickly.
- Concepts:
- Soap helps mix oil and water, forming an emulsion.
- Without soap, oil and water separate quickly.
How Soap Works
- Molecule Structure:
- Soap molecules are sodium or potassium salts of long-chain carboxylic acids.
- One end interacts with water (hydrophilic), the other with oil (hydrophobic).
- Micelles:
- Formed when soap is mixed with water.
- Hydrophobic tails are inside, trapping oil.
- Hydrophilic ends face outwards, interacting with water.
- Helps remove oily dirt, making it easy to wash away.
Activity 4.11: Soap in Hard and Soft Water
- Materials:
- 10 mL distilled (soft) water
- 10 mL hard water
- Soap solution
- Steps:
- Add soap solution to both water samples.
- Shake vigorously and observe foam formation.
- Observation:
- More foam forms in soft water.
- Hard water forms less foam and a white curdy precipitate.
Activity 4.12: Soap vs. Detergent in Hard Water
- Materials:
- Two test tubes with 10 mL hard water each
- Soap solution
- Detergent solution
- Steps:
- Add soap solution to one test tube and detergent solution to the other.
- Shake both test tubes and observe.
- Observation:
- Soap forms a curdy solid in hard water.
- Detergent forms more foam and no curdy solid.
Understanding Detergents
- Problems with Soap:
- Soap reacts with calcium and magnesium in hard water, forming scum.
- More soap is needed in hard water.
- Detergents:
- Made of sodium salts of sulphonic acids or ammonium salts.
- Do not form insoluble precipitates with calcium and magnesium.
- Effective in hard water.
- Used in shampoos and cleaning products.
Chapter Summary:
- Carbon is a versatile element.
- Carbon is the basis for all living organisms.
- Carbon is also in many things we use.
- Carbon forms many compounds because of its tetravalency.
- Tetravalency means carbon can form four bonds.
- Carbon can link with other carbon atoms; this is called catenation.
- Covalent bonds form when atoms share electrons.
- Covalent bonds help atoms fill their outermost shell.
- Carbon forms covalent bonds with hydrogen, oxygen, sulfur, nitrogen, and chlorine.
- Carbon can form double and triple bonds with other carbon atoms.
- Carbon chains can be straight, branched, or ring-shaped.
- Carbon can form a homologous series of compounds.
- In a homologous series, the same functional group is attached to carbon chains of different lengths.
- Functional groups like alcohols, aldehydes, ketones, and carboxylic acids give unique properties to carbon compounds.
- Carbon and its compounds are major sources of fuels.
- Ethanol and ethanoic acid are important carbon compounds in daily life.
- Soaps and detergents work because they have hydrophobic and hydrophilic groups.
- These groups help emulsify oily dirt and remove it.